Dezincification
Breaking the 15% Zinc Wall
Background
Some technical papers and websites on brass corrosion repeat the same caution that dezincification will occur in alloys containing more than 15% zinc. A few will go beyond that simple statement to talk about efforts to solve this issue. The 15% zinc limit is generally the only thing that stays in the reader’s mind if they are trying to design a part that is in an environment which could lead to dezincification.
Reading this was a surprise to me after spending 28 plus years as a metallurgist working with a variety of copper and brass alloys. After all, our industry had sold billions of pounds of C36000, a 35+% zinc leaded brass, to the potable water industry without corrosion problems outside of installation or grounding issues. If 15% zinc was the dividing line between a suitable brass for corrosion resistance and one prone to failure, where were the complaints and why did the plumbing industry continue to use it?
History
The reason behind this 15% zinc content concern is lost to history. Older technical papers don’t provide a trail to the problems that caused someone to establish 15% zinc as an upper limit. It just gets repeated in formal and informal articles and papers on corrosion of brass without any attribution or investigation. The problem is that “15% zinc” isn’t the full story.
The history of brass used in water applications for the last 100 years shows that brass alloys with up to and over 35% zinc have been used successfully to the tune of multiple billions of parts in the US and Europe. It didn’t happen without some effort, but it did happen as the following examples show:
1. The problem of dezincification in 70% copper 30% zinc brass tubes in steam-powered navy ship condensers was solved through an addition of an alloying element corrosion inhibitor (1);
2. The problem of severe dezincification in potable water fittings was solved in Europe in 1980 with the introduction of a brass (CZ132/CW602N) using a high temperature anneal and addition of an alloying element corrosion inhibitor to leaded brass containing 36% zinc (2);
3. In the US, alloys C36000 and C37700 with about 36% and 39% zinc respectively, were used successfully for decades without significant issues with dezincification;
4. New lead-free brasses have been developed and commercialized with zinc contents greater than 15%, some using the single or double approach of alloying element and thermal processing, to be dezincification resistant (DZR).
Development of Dezincification Resistant Brasses with Greater than 15% Zinc
It’s obvious then that brasses with zinc greater than 15% show no dezincification problems when using the metallurgical advancements of the last 100 years. The following provides explanations and data on what tools are used to make those brass alloys dezincification resistant, what is meant by dezincification resistant (DZR) and the tests that indicate they will provide long, functional lives.
I. What does dezincification resistant mean and how is it tested?
The old 15% zinc claim that brass “suffers dezincification” is vague. A brass part with zinc levels below 15% can show small levels of dezincification according to some published studies and those with up to 35% can show no dezincification. Since the problem can affect alloys with up to 15% zinc, obviously some dezincification was considered acceptable. Recently the brass world changed with the requirement to eliminate the use of leaded brass in potable water fittings (3-4) and some high-profile PEX fitting dezincification failures in the Las Vegas area (5-6). It became necessary to: 1) develop new lead-free alloys; 2) to define what “dezincification resistant” meant quantitatively (7-11); and 3) have tests that would provide guidance on how long a part can be expected to last.
According to the various standards referenced in Table 1, a brass is considered dezincification resistant (DZR) if it meets the requirements of the standards shown. There are other tests (Turner, Brandl) but the ISO6509 test has been the international standard since 1981. Independent studies (12-14) have shown this test successfully predicts long term (greater than 30 years in corrosive drinking water) performance. An important point here is that this laboratory test uses extremely harsh conditions to accelerate the potential for corrosion that will take years or decades to develop.
Table 1: Dezincification Resistance Tests and Pass/Fail Criteria
| ISO 6509(1,2 & 3) | AS2345 | UL199 | EN12164 | |
|---|---|---|---|---|
| Application | General | General | Sprinklers | Rod |
| Test Solution | 1% CuCl2 | 1% CuCl2 | 1% CuCl2 | 1% CuCl2 |
| Test Temperature | 75º C | 75º C | 75º C | 75º C |
| Test Length | 24 Hr. | 24 Hr. | 144 Hr. | 24 Hr. |
| Max. Dezinc. Depth Avg. Dezinc. Depth |
See notes 2 & 3 | — L:300µ; T:100µ |
200µ – |
100µ – |
(1) Initially developed to correlate with Swedish, Austrailian and South African field tests.
(2) Example of user defined pass/fail criteria: NSF/ANSI 14 limit is 200µ
(3) ISO 6509-2 was approved on 3/6/2017 for the following limits:
a) forgings & castings avg. 100µ, max. 200µ;
b) extruded rod longitudinal avg. 300µ, max 400µ and transverse avg. 100µ and 200µ.
What metallurgical tools are used to make alloys meet the “dezincification resistant” requirements?
There are three primary tools that make brass dezincification resistant (DZR) according to current requirements:
a. Minor alloying additions (0.20% max): Arsenic (As), Antimony (Sb), Phosphorus (P).
These are the alloying element additions that have been researched and put into production over the last 100 years. There’s enough laboratory and real-life performance data to show that they are effective in preventing dezincification in brasses with zinc contents up to 36% (Figure 1). Above 36% zinc, neither these or the major alloying elements shown can fully protect the brass because of a zinc-rich phase (beta phase) that forms during processing.
b. Major alloying additions (> 0.25%): Nickel (Ni), Tin (Sn), Aluminum (Al).
These alloying element additions have been in use longer than the minor alloying additions mentioned above. They are just as effective as the minor additions but have the same limitations of not being able to protect brasses with greater than 36% zinc because of the beta phase vulnerability to dezincification.
c. Thermal treatments such as high temperature annealing or forging:
This method reduces or eliminates the beta phase in higher zinc brasses. By converting beta phase to alpha phase, it can now be protected by the minor or major alloying additions. The precaution here is that the cooling rate after these thermal treatments has to be slow enough to prevent beta phase from reforming. Fortunately, that process is well understood by the brass mills performing the operation.
II. What data is there that shows how these metallurgical techniques improve dezincification resistance?
Figures 1 to 3 show ISO6509 test results from an independent laboratory. Figure 1 shows the range of results from having no special metallurgical protection to alloying plus thermal treatments on alloys from 5% zinc to 42% zinc. At some point above 36% zinc, beta phase begins to form increasing amounts so nothing can protect it as discussed above (Note: All test results are from Corrosion Testing Laboratories, Newark DE).
Figure 1: ISO 6509 Dezincification Depth as a Function of Zinc Content
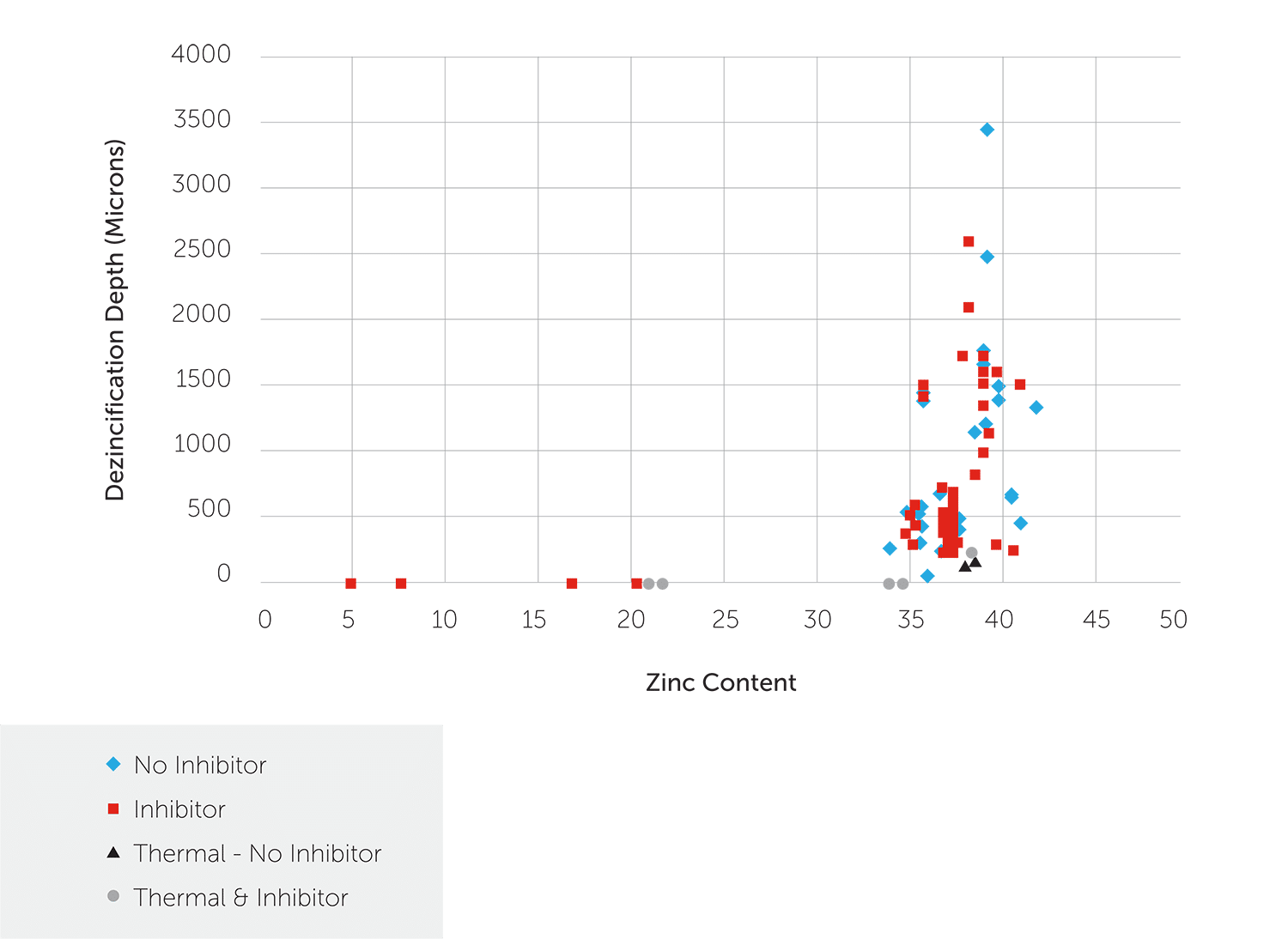
In Figure 2, you can see that the use of the two methods of the minor alloying addition and thermal treatment combine to improve dezincification resistance. However, they can only do so much before the beta phase problem negates their beneficial effects.
Figure 2: ISO 6509 Dezincification Depth (Maximum)
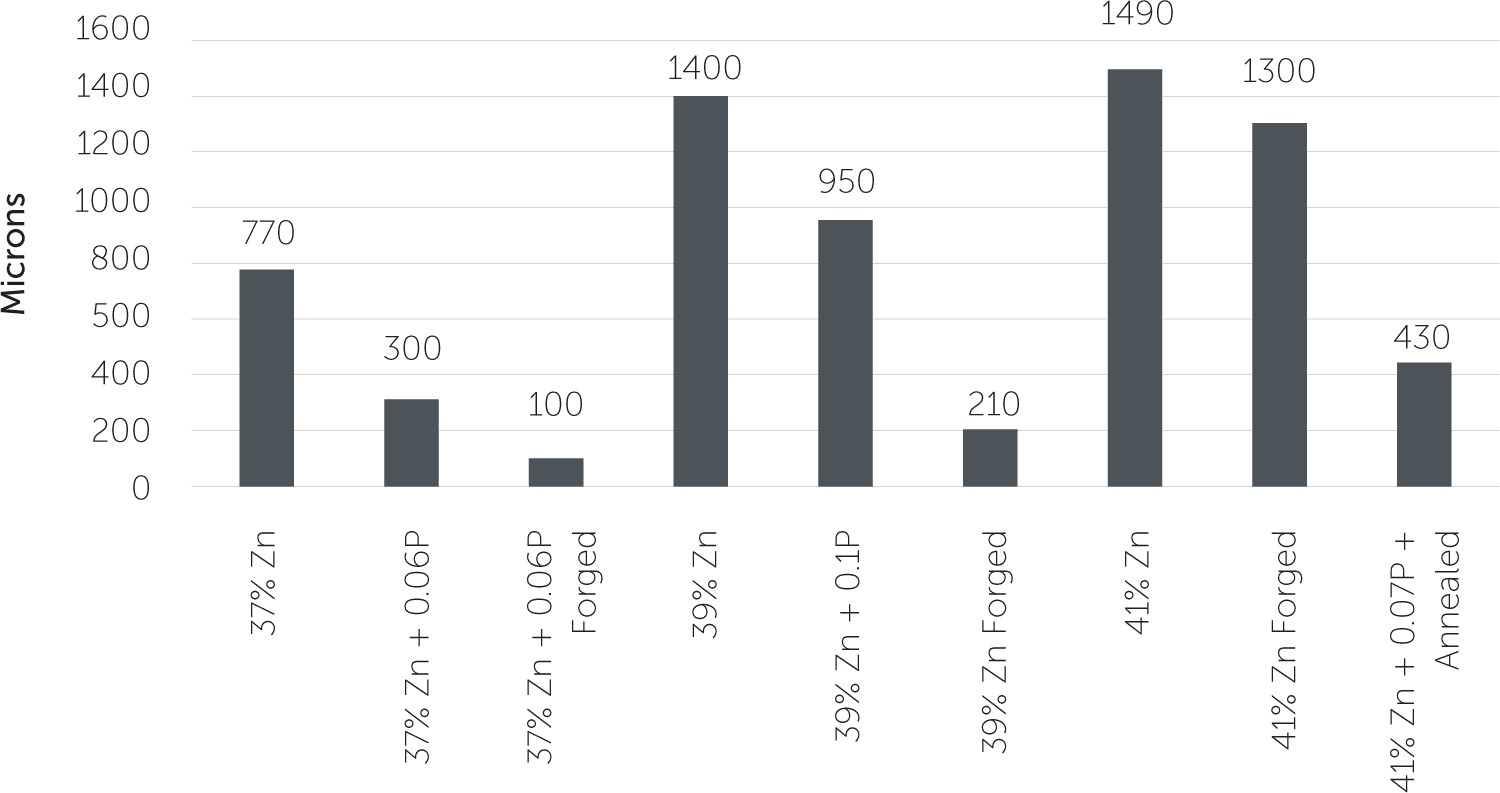
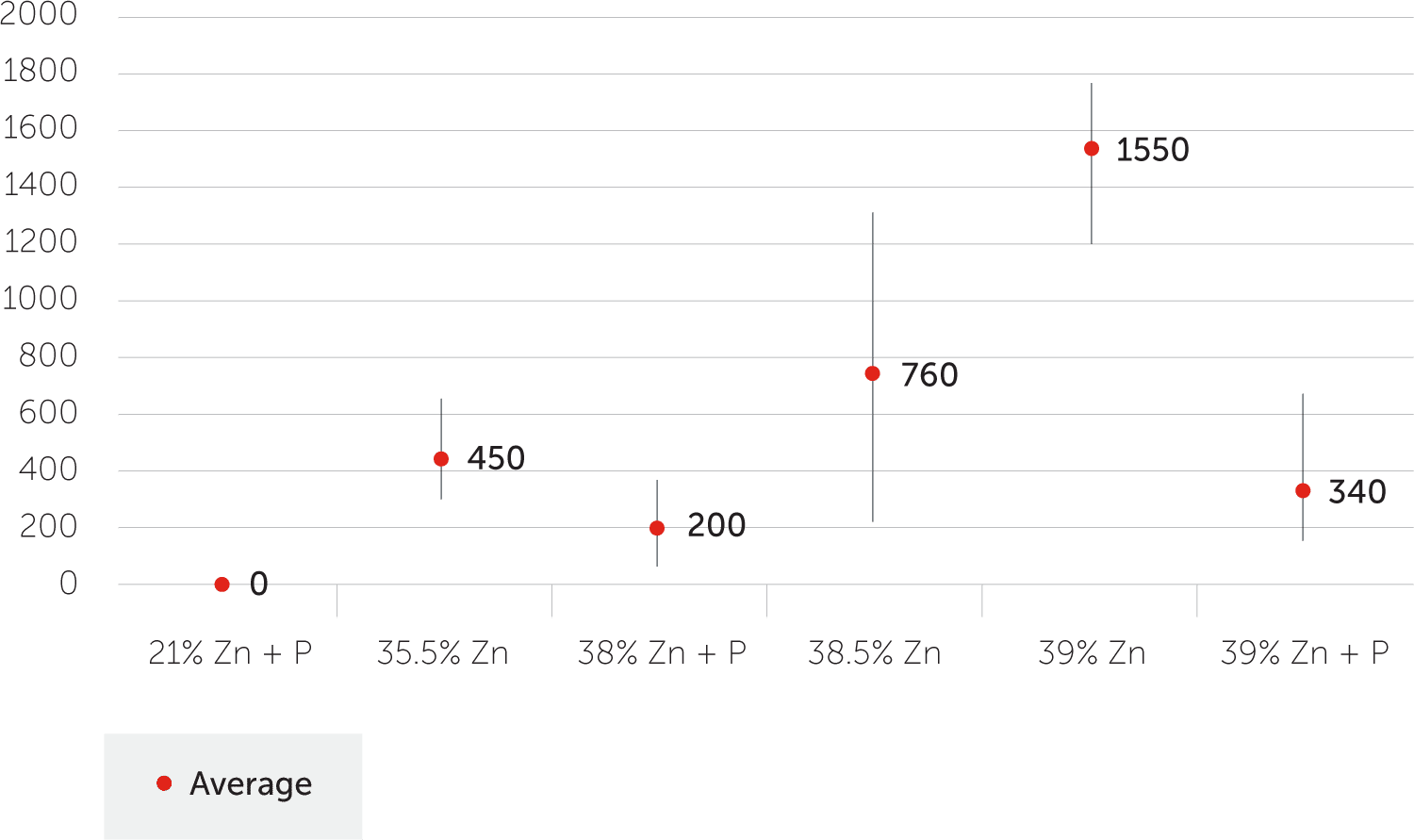
Figure 3 shows ISO6509 results for alloys with or without phosphorus and no thermal heat treatments. Except for the 21% zinc alloy, there’s a large fluctuation in results until the phosphorus addition is made.
Figure 3: Dezincification Depth (Micrometers)
Concluding Comments
The data and information above show:
1) the use of dezincification resistance alloying elements are an effective way to significantly improve dezincification resistance in brass with greater than 15% zinc;
2) publications provide evidence that theses metallurgical technologies have been in use in production brass alloys for up to 100 years;
3) any shortcomings of metallurgically advanced brasses with greater than 15% zinc would have been revealed by now;
4) this metallurgical technology has been replicated successfully among a number of “new” brasses commercialized to meet the 2014 national low lead requirement.
Thank you for your interest in reading this paper. Some of these explanations, data and more can be found in “The Basics of Dezincification” webinar.
Author,
Larry Muller
Senior Technical Advisor
Wieland Chase, LLC
July 2020
References
|
(1) Lucey, V.F., “The Mechanism of Dezincification and the Eect of Arsenic I”, British Corrosion Journal, June 1965, pp. 9-14 |
|
(2) CDA Information Sheet 36, “Dezincification Resistant Brass”, Hemel, Hempstead, England, November 1982 |
|
(3) California Assembly Bill AB1953, amended May 25, 2006 |
|
(4) US Congress Act S3874, “Reduction of Lead in Drinking Water Act”, January 5, 2010 |
|
(5) Pope, Je, “$90 million faulty plumbing settlement gets initial approval”, Las Vegas Sun, Nov. 4, 2008 |
|
(6) Sarver, E. “Insights into Non-Uniform Copper and Brass Corrosion in Potable Water Systems”, Virginia Tech, Nov. 1, 2010, page 69 |
|
(7) NSF/ANSI 14 – 2019, Section 5.9.1.3, October 8, 2019 |
|
(8) ISO6509-2, Table 1, March 6, 2017 |
|
(9) UL199 – Automatic Sprinklers for Fire Protection Service, Section 40B.1.1, July 9, 2001 |
|
(10) AS 2345 – 2006, Dezincification resistance of copper alloys, Table 1, June 13, 2006 (reconfirmed 2016) |
|
(11) DIN EN12164:2016-11, Section 6.3, April 9, 2016 |
|
(12) Saver, Edwards, Zhang, “Revisiting Dezincification Performance for Brass Plumbing Devices”, Materials Performance, Vol. 50 #5, May 2011, pg. 70-75 |
|
(13) Holm, Sundberg, Mattson, “Experiences with Brass Components for Water Installations in Sweden”, JCDA, 1982, pg. 230-238 |
|
(14) Johnson, “Evaluation of Rapid Tests for Dezincification Resistant Brass”, BNF Metals Technology Center Research Report No. A 1921, August 1977 |
The opinions expressed and conclusions reached are not intended to be professional advice. This article is for general information purposes only and Wieland Chase, LLC makes no warranty, express or implied, about the accuracy or reliability of the information presented, nor the suitability of the advice to your particular purposes. You should obtain an independent, professional opinion on the proper alloy for your intended use. Wieland Chase, LLC, its parent and affiliated companies shall not be liable for any loss or damage of whatever nature, which may arise by your use of the information from this article.
About Us
Wieland Chase is a leading brass manufacturer and supplier for brass alloys in North America. As of July 2019, Wieland Chase became part of the strong global Wieland Group with a continued commitment to safety, quality and customer service. More about the Wieland Group
Contact Us
Online Payment
14212 Selwyn Drive
Montpelier, OH 43543
p 419-485-3193
p 800-537-4291
f 419-485-5945
Copyright 2023 © Wieland.
All Rights Reserved.
Privacy Policy
Terms and Conditions
